Chemistry MCQ Quiz - Objective Question with Answer for Chemistry - Download Free PDF
Last updated on Dec 13, 2023
Latest Chemistry MCQ Objective Questions
Chemistry Question 1:
The total pressure of a mixture of ideal gases is the sum of partial pressures. This is ______ law of partial pressures.
Answer (Detailed Solution Below)
Chemistry Question 1 Detailed Solution
The correct asnwer is Dalton's.
 Key Points
Key Points
- This is known as Dalton's law of partial pressures.
- According to Dalton's law, the total pressure exerted by a mixture of non-reacting ideal gases is equal to the sum of the partial pressures of each individual gas.
- Dalton's law is based on the assumption that the gases in the mixture behave independently of one another, without any significant interactions or reactions.
 Additional Information
Additional Information
- Boyle's Law:
- Boyle's law states that, at a constant temperature, the volume of a given amount of gas is inversely proportional to its pressure.
- In other words, as the pressure of a gas increases, its volume decreases, and vice versa, provided the temperature remains constant. Mathematically, Boyle's law can be expressed as:
- P₁V₁ = P₂V₂
- where P₁ and V₁ are the initial pressure and volume, and P₂ and V₂ are the final pressure and volume, respectively.
- Charles's Law:
- Charles's law states that, at a constant pressure, the volume of a given amount of gas is directly proportional to its absolute temperature. This means that as the temperature of a gas increases, its volume also increases, and as the temperature decreases, its volume decreases, as long as the pressure remains constant. Mathematically, Charles's law can be expressed as:
- V₁/T₁ = V₂/T₂
- where V₁ and T₁ are the initial volume and temperature (in Kelvin), and V₂ and T₂ are the final volume and temperature, respectively.
- Avogadro's Law:
- Avogadro's law states that, at a constant temperature and pressure, equal volumes of gases contain an equal number of particles (atoms, molecules, or ions).
- This law implies that the volume of a gas is directly proportional to the number of moles of gas present. Mathematically, Avogadro's law can be expressed as:
- V₁/n₁ = V₂/n₂
where V₁ and n₁ are the initial volume and number of moles, and V₂ and n₂ are the final volume and number of moles, respectively.
Chemistry Question 2:
Sodium is kept in which of the following liquids?
Answer (Detailed Solution Below)
Chemistry Question 2 Detailed Solution
The correct answer is Kerosene.
 Key Points
Key Points
- Sodium cannot be kept in the open air because it is a very reactive metal.
- If it is kept in the open air, it will react with the moisture present in the air and result in the formation of sodium hydroxide (exothermic reaction)
- For this reason, it is kept in kerosene to prevent contact with oxygen and moisture.
 Additional Information
Additional Information
- Sodium (Na), chemical element of the alkali metal group Group 1(a) of the periodic table.
- Sodium is a very soft silvery-white metal.
- Sodium is the most common alkali metal and the sixth most abundant element on Earth.
- Comprising 2.8 percent of Earth’s crust.
- It occurs abundantly in nature in compounds, especially common salt Sodium chloride (NaCl)
- It forms the mineral halite and constitutes about 80 percent of the dissolved constituents of seawater.
Chemistry Question 3:
What is the relation between Celsius and Kelvin temperature?
Answer (Detailed Solution Below)
Chemistry Question 3 Detailed Solution
The correct answer is 0° C = 273 K.
 Key Points
Key Points
- The relation between Celsius and Kelvin temperature is 0° C = 273 K.
- The number of degrees between absolute zero and the freezing temperature of the water is 273 on either temperature scale.
- Therefore, the freezing temperature of the water is 273 degrees on the Kelvin temperature scale.
- While the coldest temperature possible on the Celsius temperature scale is -273 degrees.
 Additional Information
Additional Information
- The temperature conversion formula from Celsius to Kelvin is:
- K = C + 273.15
- The temperature conversion formula from Kelvin to Celcius is:
- C = K − 273.15
- The temperature conversion formula from Fahrenheit to Celsius is:
- \(C = {(F − 32) ×{ 5\over{9}}}\)
- The Temperature Conversion Formula from Celsius to Fahrenheit is:
- \(F = {C{9\over{5}}} + 32 \)
- The Temperature Conversion Formula from Fahrenheit to Kelvin is:
- \(K = {(F − 32) × {5\over{9}} + 273.15}\)
Chemistry Question 4:
The chemical name of 'Vitamin A' is
Answer (Detailed Solution Below)
Chemistry Question 4 Detailed Solution
- Retinol is the fat-soluble vitamin retinol.
- The chemical name of Vitamin A is Retinol.
- Deficiency of vitamin A causes night blindness.
- Vitamin A can be found in many fruits, vegetables, fish and dairy products
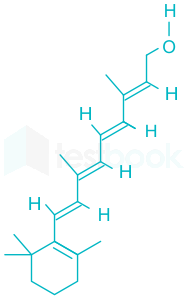
Thus, we can conclude that the chemical name of 'Vitamin A' is Retinol.
 Additional Information
Additional Information
| Vitamin | Chemical Name |
| Vitamin A | Retinol |
| Vitamin C | Ascorbic Acid |
| Vitamin B1 | Thymine |
| Vitamin B2 | Riboflavin |
| Vitamin B3 | Niacin |
| Vitamin B5 | Pantothenic acid |
| Vitamin B7 | Biotin |
| Vitamin C | Ascorbic acid |
| Vitamin D | Cholecalciferol |
| Vitamin E | Tocopherol |
Chemistry Question 5:
'Galvanisation' is a process in which
Answer (Detailed Solution Below)
Chemistry Question 5 Detailed Solution
 Key Points
Key Points- When iron comes in contact with air and moisture, rust forms over it and eats away the metal.
- Rust is hydrated iron oxide with the chemical formula Fe2O3. nH2O.
- Zinc is coated over iron to protect the latter from rusting.
- The most common way of galvanizing iron is dipping it in the hot molten zinc.
- When Zinc gets coated over the iron it protects iron from coming in contact with air and moisture.
Hence, we can conclude that 'Galvanisation' is a process in which Zinc is deposited as layer on iron.
Top Chemistry MCQ Objective Questions
Chemical name of washing soda is:
Answer (Detailed Solution Below)
Chemistry Question 6 Detailed Solution
Download Solution PDFThe correct answer is Sodium carbonate.
Explanation:
- Washing soda is a chemical compound with the formula Na2CO3, known as sodium carbonate, and it's a salt of carbonic acid.
- Properties of a Washing soda:
- It is a transparent crystalline solid.
- It is one of the few metal carbonates which are soluble in water.
- It is alkaline with a pH level of 11, it turns red litmus to blue.
- It has detergent properties or cleansing properties because it can remove dirt and grease from dirty clothes, etc.
- It attacks dirt and grease to form water-soluble products, which are then washed away on rinsing with water.
 Important Points
Important Points
Some common chemical compounds with their common names are:
|
Chemical Compounds |
Common Names |
Chemical Formulas |
|
Sodium Bicarbonate |
Baking Soda |
NaHCO3 |
|
Calcium ChlorohypoChlorite |
Bleaching Powder |
CaOCl2 |
|
Sodium Hydroxide |
Caustic Soda |
NaOH |
|
Sodium Carbonate |
Washing Soda |
Na2CO3 .10 H2O |
|
Carbon Dioxide |
Dry Ice |
CO2 |
|
Copper Sulphate |
Blue Vitriol |
CuSO4 |
|
Ferrous Sulphate |
Green Vitriol |
FeSO4 |
|
Sulphuric Acid |
Oil of vitriol |
H2SO4 |
|
Calcium Sulphate Hemihydrate |
Plaster of Paris |
(CaSO4. 1/2H2O) |
|
Calcium Sulphate Dihydrate |
Gypsum |
CaSO4.2H2O |
|
Calcium Hydroxide |
Slaked Lime |
Ca(OH)2 |
|
Chile Saltpeter |
Sodium nitrate |
NaNO3 |
|
Saltpetre |
Potassium nitrate |
KNO3 |
|
Muriatic acid |
Hydrochloric acid |
HCl |
Which of the following is called 'Pearl ash'?
Answer (Detailed Solution Below)
Chemistry Question 7 Detailed Solution
Download Solution PDFK2CO3 or potassium carbonate is known as pearl ash.
- Pearl ash, in ancient times, was created by baking potash in a kiln in order to remove impurities. The remaining fine, white powder was pearl ash.
- Potassium carbonate is an inorganic compound and a white salt which is soluble in water.
- It is mainly used in the production of glass and soap.
 Additional Information
Additional Information
Which acid is present in sour milk?
Answer (Detailed Solution Below)
Chemistry Question 8 Detailed Solution
Download Solution PDFThe correct answer is Lactic Acid.
 Key Points
Key Points
- Lactic acid is present in Sour milk or curd.
- The sourness of the milk is due to the presence of lactic acid.
- Human beings feel tired due to the accumulation of lactic acid in the muscles.
 Additional Information
Additional Information
| Natural source | Acid |
| Vinegar | Acetic acid |
| Orange | Citric acid |
| Tamarind | Tartaric acid |
| Tomato | Oxalic acid |
The ratio of the mass of hydrogen to the mass of oxygen in water is always ________.
Answer (Detailed Solution Below)
Chemistry Question 9 Detailed Solution
Download Solution PDF Key Points
Key Points
- Atomic Mass of the Hydrogen = 1
- Atomic Mass of the oxygen = 16
Explanation:
1 mole of hydrogen = 1gm
1 mole of oxygen = 16gm
Water (H2O) = 2 Hydrogen atoms + 1 Oxygen atom
2 mole of hydrogen = 2gm
1 mole of oxygen = 16 gm
The ratio of the mass of Hydrogen: Ratio of mass of Oxygen = 2/16 = 1/8
The ratio of the mass of Hydrogen to the mass of Oxygen in water is always 1:8.
How many water molecules are present in one molecule of washing soda?
Answer (Detailed Solution Below)
Chemistry Question 10 Detailed Solution
Download Solution PDF- The number of water molecules present is washing soda is 10.
- We know the molecular formula for Washing Soda is Na2CO3.10H2O.
- Recrystallisation of Sodium Carbonate (Na2CO3) gives washing soda.
- In a Washing soda, water is present in the form of crystals.
What is the common name of Mercury Sulfide?
Answer (Detailed Solution Below)
Chemistry Question 11 Detailed Solution
Download Solution PDFThe correct answer is Vermilion.
- Mercury Sulfide is also known as Vermilion.
- It is a chemical compound composed of the chemical elements mercury and sulfur.
- The chemical formula of Mercury sulfide is HgS.
- It is dimorphic with two crystal forms:
- Red cinnabar
- Black metacinnabar
 Additional Information
Additional Information
- Marsh Gas is the common name of Methane with the formula of CH4.
- Mohr's Salt is the common name of Ammonium Ferrous Sulphate with the formula of (NH4)2Fe(SO4)2(H2O)6.
- Potash Alum is the common name of Potassium Aluminium Sulphate with the formula of KAl(SO4)2.
CO2 when passed in excess, in lime water turns colourless again because of:
Answer (Detailed Solution Below)
Chemistry Question 12 Detailed Solution
Download Solution PDFExplanation:
- Calcium hydroxide is sparingly soluble in water producing an alkaline solution known as limewater.
- Calcium Carbonate is a chemical compound found commonly in rocks as minerals and is the main component of pearls and the shells of marine organisms, eggs, etc.
- When carbon dioxide gas is passed through or over limewater, it turns milky due to the formation of calcium carbonate.
- In the chemical reaction it can be shown as :
\(\rm \underset{Lime\ water}{Ca (OH)_2} \ (aq) \ + \ \underset{Carbon \ Dioxide}{CO_2 \ (g) }\ \longrightarrow \ \underset{Calcium \ Carbonate}{CaCO_3 \ (g)}\)
- However, when an excess of CO2 is passed through this solution, the milkiness disappears. This is due to the formation of calcium bicarbonate which is colorless and soluble in water.
 Additional Information Reaction involved-
Additional Information Reaction involved-
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
- The evolved gas is carbon dioxide which then passes through lime water and turns it milky.
Ca(OH)2 + CO2 → H2O + CaCO3
- Due to formation of these compounds
- when excess CO2 is passed
CaCO3 + H2O + CO2 → Ca(HCO3)2
- Bicarbonate is formed which again clears the solution
 Mistake Points
Mistake Points
- Do not confuse Calcium carbonate and calcium bicarbonate.
- One produces white colour while the other makes it colourless.
To which category of reactions does the following chemical reaction belong?
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
Answer (Detailed Solution Below)
Chemistry Question 13 Detailed Solution
Download Solution PDFThe correct answer is Double displacement reaction.
Concept:
Chemical reaction:
- It is a process in which one or more substances, the reactants, are converted to one or more different substances, the products.
- A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
-
Double displacement reaction:
- It is a chemical reaction in which two compounds react and exchange their ions forming new products.
- This reaction often results in the formation of an insoluble compound called a precipitate.
- Such reactions are represented by equations of the following form: A+B- + C+D- → A+D- + B-C+
In the given chemical reaction:
NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
The reactants NaCl and AgNO3 have exchanged their ions to form NaNO3 and AgCl.
Thus, it is an example of a double displacement reaction.
 Additional Information
Additional Information
Types of Chemical Reactions:
Combination reaction:
- Two or more elements or compounds combine together to form a single compound.
- Such reactions are represented by equations of the following form: A + B → AB.
Decomposition reaction:
- The opposite of a combination reaction, a complex molecule breaks down to make simpler ones.
- Such reactions are represented by equations of the following form: AB → A + B.
Displacement reaction:
- One element takes place with another element in the compound.
- Such reactions are represented by equations of the following form: A + BC → AC + B.
Redox reaction:
- It is a reaction in which one species is reduced and the other is oxidized.
- Reduction means losing Oxygen or gaining hydrogen
- Oxidation means gaining oxygen or losing hydrogen.
What is pH of Lemon Juice?
Answer (Detailed Solution Below)
Chemistry Question 14 Detailed Solution
Download Solution PDFpH of some common aqueous solutions
| Solution | pH |
| 1M HCl | 0.0 |
| Gastric juice | 1.0 |
| Lemon juice | 2-3 |
| Vinegar | 3.0 |
| Tomato juice | 4.1 |
| Pure water, sugar solution | 7.0 |
Which of the following metals reacts with steam to form a metal oxide and hydrogen?
Answer (Detailed Solution Below)
Chemistry Question 15 Detailed Solution
Download Solution PDF- Aluminium (Al) metal reacts with steam to form a metal oxide and hydrogen.
- This reaction can be given as
2Al + 3H2O (g) → Al2O3 + 3H2 (g)
- Other metals of this type are Iron (Fe) and Zinc (Zn).
- But these metals do not react with either hot or cold water.


